Products
ILC Cells
ILC Cells kit contains islet like cells and supplement media. This product has been specially formulated to contain cryopreserved single cells that are frozen in vials. The islet like cells in this kit are derived from human iPSC and closely resemble human cadaveric islets from a morphological and functional perspective.
In vitro cellular models such as cadaver derived beta islet cells have been an important part of Diabetes research, but they are constrained by many factors such as donor to donor variability, inconsistent availability and high costs. RMS developed the ILC Cells kit to ensure that the research community can readily access islet like cells that share morphological and functional similarities with human beta islet cells. By producing these cells from human iPSC cell lines, we have a scalable production capacity, consistent cell quality, on-demand supply and reasonable cost. ILC Cells kit is intended for diabetes research applications such as in vitro cell studies, drug development applications such as drug discovery and toxicity screening.
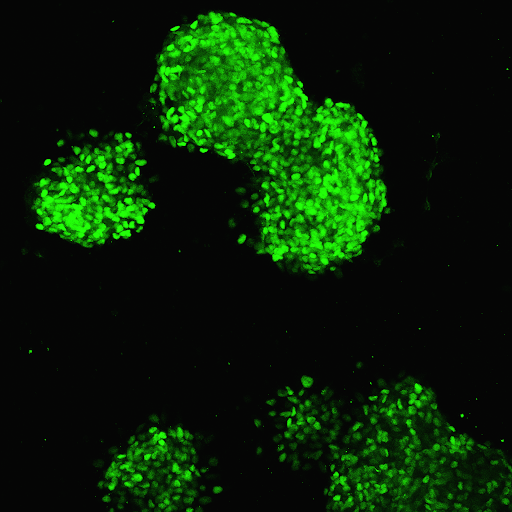
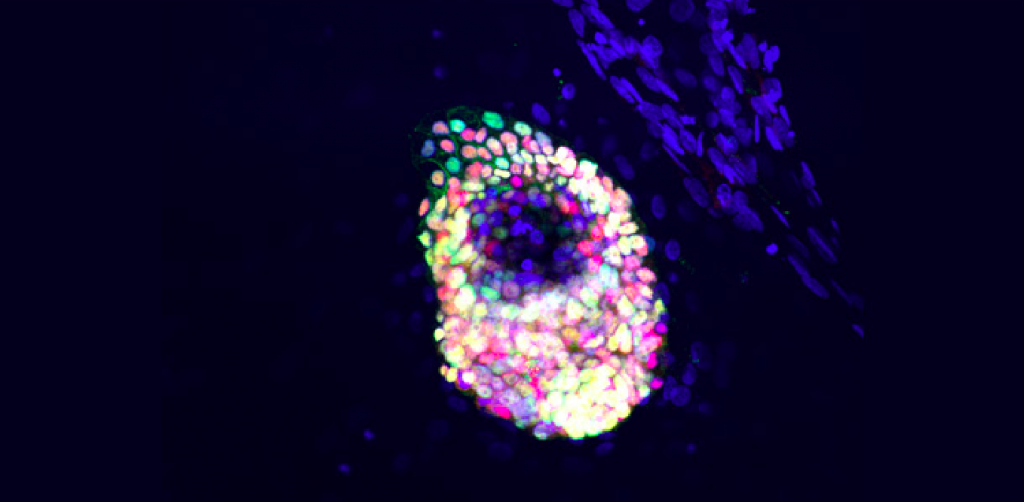
ILC Cell Therapy Candidate
We are developing autologous and allogeneic versions of ILC cellular therapeutic. In the case of autologous ILC cellular therapeutic we will convert the diabetes patients own cells into iPSC cells followed by differentiation of the patient derived iPSC into ILC using our patented and proprietary technology. These patient cell derived ILCs will then be surgically transplanted into the patient’s body. Our allogeneic version of ILC cellular therapeutic is derived from human iPSC cell line manufactured using GMP process. This GMP human iPSC cell line is differentiated into ILC’s using our technology and subsequently transplanted into the patient’s body. Both approaches have the potential to cure type 1 and type 2 diabetes, since the transplanted ILCs will begin to function like the pancreas, by physiologically regulating glucose and insulin levels in the patient’s blood. Both ILC cellular therapeutic candidates are in pre-clinical development being evaluated in the laboratory and in animal models. We are working towards a phase I clinical trial to assess the safety of our ILC cellular therapeutic candidate in patients with diabetes.